CuOTf and the 6π e- Electrocyclization of Trienes |
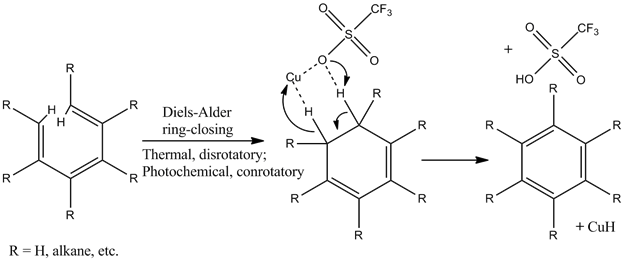
| Aromaticity of a cyclic system is an extremely stable state for a compound to be in. Reactions in which aromaticity or electrocyclization is created or eliminated see their equilibriums strongly favor the highly resonance-stabilized species. Because aromaticity is so strongly favored, aromatic portions of compounds are typically unreactive to a large collection of treatments that would normally react with one or many double bonds, conjugated or not. Trienes are compounds that have three double bonds. Trienes that have three consecutive alternating double and single bonds (i.e. d, s, d, s, d) are conjugated and exhibit a number of resonance structures, an important precursor to aromaticity. It is important that trienes should have sufficient flexibility for their ends to reach one another. Although sterically and conformationally unfavorable, in the case that trienes go through an intramolecular Diels-Alder ring-closing reaction, a cyclic diene results. Stereochemistry at the carbons that were previously the ends of the triene is variable, depending on whether the ring-closing was thermal or photochemical. Those two conditions give enantiomeric products in relation to itself, and diastereomeric products in relation to each other. The cyclic diene that results can become aromatic with the aid of the reagent CuOTf, which is a copper-trifluoromethanesulfonate ligand. The copper-oxygen bond is part ionic, but also more covalent. The two atoms coordinate to capture two hydrogens and a lone pair from the cyclic diene. The oxygen picks up a proton while the copper picks up a hydride, and as the final electron pair shifts to p-orbitals, a 6π electron system is created and the ring becomes aromatic. |
 |
